Click Here to Download PDF Version
Telehealth Newsletter
Official Newsletter of Tamil Nadu Chapter of Telemedicine Society of India
|
What is New? In the April issue of the newsletter we have covered some interesting articles including one by Prof. K. Ganapathy on ‘Metaverse in Healthcare; that will eventually perhaps lead to you having a Metaverse Avatars visiting different virtual worlds. Ayushman Bharat Digital Mission has invited Consultation Paper on creating Drug Registry for the country and the last date for such comment submission is midnight of 1st May. Please do spend some time by visiting the website and sending your valuable suggestion on this very important initiative. The COVID numbers are again showing a rise, so we request our readers to continue wearing masks, practice hand wash and keep physical distancing. The course of the disease can still be unpredictable so do take your precautions. Thank You |
Consultation Paper on Drug Registry by Ayushman Bharat Digital Mission
Ayushman Bharat Digital Mission (ABDM) has been launched to create a national digital health ecosystem that supports universal health coverage in an efficient, accessible, inclusive, affordable, timely, and safe manner.
ABDM envisions open, interoperable, standards-based digital systems, and ensures the security, confidentiality, and privacy of health-related personal information.
Registries are one of the core building blocks of the ABDM which if standardized would help in enabling interoperability of healthcare data. These registries shall be designed with strong data governance mechanisms, adhering to the principles of verifiability, accessibility, and identity management.
One of the critical components of these registries is the Drug registry, which is envisioned to be a single, up to date, recognized registry of all the drugs. It is being conceptualized as the primary source of information for all other databases and lists and facilitates the exchange of standardized data across all systems of medicine, from allopathy to Ayurveda.
A central database of the approved drugs sold in the market will serve multiple benefits including free availability of verified information for all drugs, simplified regulatory flows, smoother supply chain management, streamlined insurance claim processing, innovations in clinical decisions, disease management and assurance models. Initially, the goal is to comprehensively capture relevant & accurate details of all drugs sold in India.
Over time, the drug registry is envisaged to help smoothen the inventory flow throughout the drug supply chain, improving the quality and patient trust and ultimately enabling patient centric digitization by ensuring machine readability of prescribed drugs. This document covers the strategic and technical design associated with the Drug Registry. The potential sources of input data, self-certification, verification, and data distribution flow have been proposed in the document to ensure the creation of a single nationally recognized source of truth for data on drugs that is trusted, digitally enabled, and widely adopted by the healthcare ecosystem stakeholders. We look forward to feedback and support from the ecosystem partners to enable the design and adoption of a Drug registry in India.
Written Comments on the Consultation Paper are invited from all the stakeholders by 11:59 pm of May 1, 2022.
Comments are to be preferably provided electronically on the NDHM website via form available at https://abdm.gov.in/home/Publications
The comments may also be sent on the email ID: abdm@nha.go
National Health Authority Tower
1, Jeevan Bharati Building, Connaught Place, New Delhi – 110001.
 Webinars on Digital Health and its Future
Webinars on Digital Health and its Future
Prof. K. Ganapathy
Past President, Telemedicine Society of India & Neurological Society of India | Hon Distinguished Professor The Tamilnadu Dr. MGR Medical University | Emeritus Professor, National Academy of Medical Sciences | Formerly Adjunct Professor IIT Madras & Anna University | Director Apollo Telemedicine Networking Foundation & Apollo Tele Health Services | URL: www.kganapathy.in
On Thursday 24th March 2022 the Tamilnadu Chapter of the Telemedicine Society of India joined hands with PALS as co-organisers to promote a two webinars on
1. Role of technology in enabling Digital Health
2. Role of Technology in Health Care in 2030 – A peep into the future.
This 2 hour interactive session was conducted by Prof. K. Ganapathy Past President TSI.
The webinars were conducted on 24th March 2022 and it marked the 22nd anniversary of the formal commissioning of the world’s first VSAT enabled village hospital @ Aragonda the birth place of Dr Prathap C Reddy Founder Chairman of the Apollo Hospitals Group. To commemorate this a global webinar was held. The recorded proceedings are available @ https://youtu.be/eG2yj1pBkjU or https://bit.ly/3vLc257
The contents were customised specifically for students and faculty of Engineering Colleges.
PALS is an educational initiative by volunteers from Alumni Fraternity of various IIT’s, for the benefit of students of engineering colleges (https://palspgm.com). The two talks are available @ PALS T2P VIDEO RECORDING-24.03.22 or https://youtu.be/S1SPl5B0fq4

The Metaverse is slowly becoming an interesting and popular topic. On 6th March 2022 the Chennai Chapter of the COMPUTER SOCIETY OF INDIA had a 2 hour session on The Metaverse. This included a talk on “Role of the Metaverse in Healthcare” by Prof. K. Ganapathy Advisor to the Tamilnadu Chapter of the TSI. Dr Ganapathy defined the Metaverse as the augmented Virtual World derived by convergence of virtual and physical space, where users can interact within the augmented world, to meet each other virtually, immersing themselves in performing virtual activities that gives real experiences. The untapped potential in healthcare to combine AI, Virtual Reality, Augmented VR, Extended VR, Internet of Medical Things , Web 3.0, I Cloud, Edge, Quantum Computing and Robotics was illustrated.
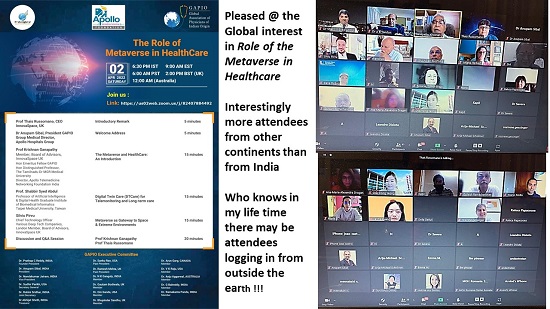
On 2nd April 2022 Innovaspace, GAPIO ( Global Association of Physicians of Indian Origin ) and Apollo Telemedikcine Networking Foundation jointly organized an international webinar on Role of the Metaverse in Healthcare. The speakers included Dr Shabbir Sayeed Abdul Professor of AI & Digital Health, Taipei and Mr Silviu Pervu CTO Various Deep Tech Companies, London, besides Dr Ganapathy. Interestingly there were more attendees from other countries than India !! The talks are available @ https://bit.ly/3seWx4P

 Decoding the Healthcare Professional Registry’s Utility in Telemedicine
Decoding the Healthcare Professional Registry’s Utility in Telemedicine
Anay Shukla
Founding Partner, Arogya Legal – Health Laws Specialist Law Firm
Eshika Phadke
Associate, Arogya Legal – Health Laws Specialist Law Firm
Saloni Kedia
Associate, Arogya Legal – Health Laws Specialist Law Firm
The Ayushman Bharat Digital Mission (ABDM) was launched in 2020 with the vision of creating “a national digital health ecosystem that supports universal health coverage” and “establishing registries at the appropriate level to create a single source of truth in respect of clinical establishments, healthcare professionals, health workers, drugs and pharmacies”.
To streamline the flow of health data, the ABDM also envisages four electronic registries: the Ayushman Bharat Health Account (ABHA), the Healthcare Professionals Registry (HPR), the Health Facility Registry (HFR), and the Drug Registry. The ABHA is a unique identification number that will be assigned to each individual patient, and enables them to digitise their health records and make it available to healthcare professionals. The HPR will be a registry of all doctors, nurses, allied and healthcare professionals, paramedical staff, ASHAs, etc across both the modern and traditional systems of medicine. The HFR will serve as a directory of all public and private hospitals, clinics, diagnostic centres, pharmacies, etc. The Drug Registry will be a database of all drugs from all systems of medicine that are approved in India.
Recognising the role that telemedicine plays in the realisation of the vision, the ABDM also places a strong emphasis on creating an open telemedicine network, which will consist of a government owned, operated and managed system at the back-end, and privately owned and operated consumer apps at the front-end which operate on the data contained in the four registries. In this article, we will focus specifically on the HPR, and its significance in telemedicine.
Doctors are familiar with the rolls that are maintained by the National Medical Commission and the all the state medical councils – the HPR is essentially a larger scale version of the list, which will include all professionals involved in the healthcare delivery system, and will enable registered professionals to be connected to the ecosystem. Doctors may elect to enrol with the HPR and, upon verification by the respective medical council, they will be given access to the account and will be assigned a Healthcare Professional ID. For a professional providing teleconsultation, there are several advantages to being enrolled in the HPR.
First, a profile on the platform will be generated which will contain the doctor’s name, specialisation, educational background, availability, etc. As there is no law that explicitly recognizes the right of the doctors to be listed on the private aggregator platforms, the creation of a profile under the government-led HPR will provide online visibility to the doctor without there being any apprehension of violation of law.
Second, as per the Telemedicine Guidelines, doctors providing teleconsultations are required to establish a mechanism whereby patients can verify the doctor’s credentials and contact details. Thus, even when a doctor is providing consultations via phone calls, he/she can lead the patient to their public profile on the HPR, which will serve as a credible source for verification.
Third, the ecosystem will enable doctors to digitise their in-clinic practice and adopt paperless diagnostic reports, discharge summaries, prescriptions, etc which can be e-signed. This will provide for a seamless hybrid practice where the doctor can consult patients both in-clinic and through a teleconsultation, as per the patient’s convenience, without having to assume additional difficulties with documentation and accessing records.
Fourth, the doctor will have access to all the historic records of patients who have opted for the ABHA scheme and consented to have their data shared. This will reduce the dependence on the patient to furnish their records to enable the doctor to gain a holistic understanding of the patient’s condition prior to proceeding with a diagnosis and treatment plan. As per Telemedicine Guidelines, doctors are required to ensure that they have all the information required as per standard treatment guidelines before proceeding with a treatment plan for a patient. Access to past records will help doctors meet this requirement.
Finally, the drug registry (once operationalised) will offer access to a single, up-to-date, centralized repository of all the drugs across all systems of medicine which are approved and are available in the Indian market, which will provide a treasure trove of information that will help doctors while issuing prescriptions.
The implementation of the digital health ecosystem is presently still at a primitive stage, and the full potential of it is yet to be realised. That being said, it is clear that it will prove highly advantageous for doctors who are looking to take or grow their online practice. Thus, while enrolment with the HPR is voluntary, it may be a pragmatic step to take for doctors who offer teleconsultation.
Further reading
- Consultation Paper on HPR (Accessible here)
- FAQs about the HPR (Accessible here)
-
Telemedicine Practice Guidelines (Accessible here)
 TELEMEDICINE IN INDIA: A CRITICAL ANALYSIS OF THE EXISTING LEGAL FRAMEWORK – A Summary
TELEMEDICINE IN INDIA: A CRITICAL ANALYSIS OF THE EXISTING LEGAL FRAMEWORK – A Summary
Dr. Karan Shekar MBBS, PGDMLE (NLSIU)
Resident, Department of Cardiothoracic Surgery, Narayana Health, Bangalore.
Contact: +91-9108158715/+91-9110622903,Email ID: drkaranshekar@gmail.com
Dr. Arpita H.C,
Assistant Professor,Centre for Health Law and Ethics,
National Law School of India University,Bengaluru. Email ID: arpithahc@nls.ac.in
Introduction:
Healthcare sector is an 8.6 trillion dollar industry having major economic influence in our developing country. However, India still ranks 145th out of 195 countries in terms of quality and accessibility of healthcare. The calling of WHO for “Universal Health Coverage – Health for everyone, everywhere” has a huge potential to be realized through the recent implementation of Telemedicine in India. The author in this paper attempts to summarize the Critical analysis of telemedicine guidelines article from UPES Law review: Vol. VI 2021, ISBN 2347-9620; which dwells on effective regulatory Framework for Telemedicine implementation.
Telemedicine has existed since decades. From the early 1990s, due to a rapid rise in the usage of the internet brought about a huge dependence on telemedicine for immediate healthcare assistance and has been utilized, however without legal framework. In different countries practicing telemedicine, there were hardly any standards for medical practitioners to follow and patients to expect during a teleconsultation process. Issues such as personal data privacy, security, lack of a legally accepted prescription, lack of doctor credentialing, and liabilities, standard operating protocols, malpractice and negligence, duty of care were paramount. Without addressing them with statutory guidelines, it was impractical to implement the practice of telemedicine to serve the country’s healthcare needs.
During the peak of COVID pandemic, much to the relief of many healthcare professionals, the Ministry of Health and Family Welfare in partnership with NITI Aayog released the “Telemedicine Practice Guidelines” which therefore legalized the practice. The guidelines are patient-friendly but comprehensive and cater to the interests of all the concerned stakeholders, including the RMP, patient, and technology and cuts across all digital platforms
Critical Analysis of the guidelines:
- Cross jurisdictional Consultations:
Having provided different opinions regarding the practice of medicine among different states as observed by the high court in two separate occasions12 puts the guidelines in ambiguity regarding the inter-state practice of telemedicine. - Registration of Complaints:
The ambiguity as to where to register a complaint would bother all three parties i.e. The RMP, the patient and the telemedicine provider. If the patient wishes to register a complaint against a doctor for negligence, there is no clear guideline as to where should the patient place the complaint. Would the complaint be taken up at the location where the patient resides or whether it is at the doctor’s location or is it at the location from where the telemedical platform operates? - Patient confidentiality and Data privacy:
The guidelines are clear that the patient may use any platform to connect with the doctor for a teleconsultation. It mentions the use of WhatsApp, Facebook messenger, Google Hangouts, Skype, email, fax, etc. However, due to lack of contract between parties providing such mediums and the healthcare practitioner, there is high chance of data breach with sensitive medical information. - Incorporation of these guidelines into public healthcare:
Ensuring that the benefit of telemedicine reaches the corners, institutional incorporation is necessary. - Research and Evaluation:
As practitioners of modern medicine, RMPs believe in evidence-based medicine. The novel clinical practice is cost-effective and convenient. Yet, it must also be proved to be safe, efficient, based on empirical, unprejudiced, and convincing evidence.
Recommendations to the guidelines:
- Institution of a regulatory authority:
Establishing a competent, independent authority to oversee the process of telemedicine in general digital health is essential. A pre-existing competent authority such as Telemedicine society may overlook the needs and demands of this novel practice. - Institutional incorporation:
Telemedicine should be a department of its own in all government primary health centers, community health centers, and tertiary care hospitals. - Telemedicine in healthcare curriculum:
Medical schools and nursing schools should develop comprehensive telehealth curricula, including lecture series, clinical clerkships, and rotations. - Licensure to practice telemedicine:
Teleconsultants should be verified by a single authority undergoing uniform training, and certification process for the practice. They must be allowed practice without cross-border jurisdiction which creates an obstacle in delivering teleconsultations. - Accreditation and audit:
A competent authority can ensure access, affordability, efficiency, quality and effectiveness of the practice of telemedicine. Regular audits and certification stimulates continuous improvement within the organization. This too, can be overseen by the Telemedicine society. - Insurance coverage:
All insurance companies, government, and private must recognize and acknowledge telemedicine and adopt policies emphasizing telemedical care for ease of healthcare access to the public. - Technical aspects:
A separate technology department with a sole focus on tele-medical services for data privacy and protection needs to be instituted. - Research in Telemedicine:
Enabling research and funding for research to advance implementation, resource utilization, identify populations with increased healthcare demands, quality improvement, and clinical outcomes. - Furthering telemedicine:
Exploring opportunities for utilizing telemedicine by forming partnerships with interdepartments or centers of telemedicine to increase access to expert clinical advice from specialist doctors. - Public awareness:
Ministry of health associated with its healthcare functionaries to promote and educate people to embrace telemedicine for all primary consultations and to minimize visits to hospitals.
*Complete Article is available at UPES Law review: “Telemedicine in India: A critical analysis of the existing legal framework.” Vol. VI 2021, ISBN 2347-9620
1Malay Ganguly V. Medical Council of India. Writ Petition (C) No. 317 of 2000.
2The Federation of State Medical Boards; Telemedicine Policies Board by Board Overview, Telemedicine Licensure. Available at:
https://www.fsmb.org/siteassets/advocacy/key-issues/telemedicine_policies_by_state.pdf
Telemedicine – News from India & Abroad
Artificial Intelligence (AI) Helps Detect Cancer from Patient Data Securely
Researchers developed a novel way of using artificial intelligence (AI) to predict cancer from patient data without putting personal information at risk…..Readmore
Robots may Now Mimic Humans in Performing Tasks
Robots can now adapt to their working methods to solve complex tasks — similar to humans as per a study at the Chalmers University of Technology, Sweden, presented at the robot conference IROS 2021……Readmore
Click here to Become a Member of Telemedicine Society of India
Telemedicine Practice Guidelines – A Foundation Course for RMPs by TSI Faculty
To know more about the Telemedicine Foundation Course click on the link below:
https://tsitn.org/tpg-course/
TN – TSI invites all the TSI Chapters and Members to submit information on their upcoming Webinar or Events (50 words), News related to Telemedicine (200 words) or short articles (500 words) for the monthly e-newsletter.Guidelines for submission to TN TSI Newsletter-
|
Submission may be sent to – tsigrouptn@gmail.com
Editors reserve the rights for accepting and publishing any submitted material.
Editor in Chief – Dr. Sunil Shroff
Editors – Dr. Senthil Tamilarasan & Dr. Sheila John
Technical Partner- https://www.medindia.net


 Webinars on Digital Health and its Future
Webinars on Digital Health and its Future Decoding the Healthcare Professional Registry’s Utility in Telemedicine
Decoding the Healthcare Professional Registry’s Utility in Telemedicine TELEMEDICINE IN INDIA: A CRITICAL ANALYSIS OF THE EXISTING LEGAL FRAMEWORK – A Summary
TELEMEDICINE IN INDIA: A CRITICAL ANALYSIS OF THE EXISTING LEGAL FRAMEWORK – A Summary